Research Projects
Organ-on-a-chip model of breast cancer bone metastases
| Principal investigator: | Martin KNIGHT (Queen Mary University of London) |
| Co-investigator(s): | Stefaan VERBRUGGEN (Queen Mary University of London) |
| Funding source: | CRUK and EPSRC |
| Value: £268,700 | |
| Start: 01-12-2020 / End: 31-05-2023 |
Background
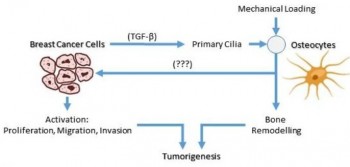
A common site for invasive ductal carcinomas (IDC) metastasis is bone, affecting about 70%
of patients. Once metastasis to bone has occurred the five-year survival rate drops from
99% to 29%. How breast cancer metastasises to bone is poorly understood, partly because
of the lack of appropriate models. Organ-on-a-chip technology is a new branch of
bioengineering which may be used to accurately recapitulate bone metastasis by combining
multiple cell types in a microfluidic chip with circulating media and physiological
biomechanical forces. However a reliable breast cancer bone metastasis chip is currently not
available.
Aims
The aim of this study is to develop an organ-on-a-chip model of bone metastasis in breast
cancer and to use this to test the hypothesis that the interaction between cancer cells and
osteocytes controls the metastatic niche and tumour development. We hypothesize that this
process is regulated by physiological biomechanical loading of bone and osteocyte primary
cilia.
Methods
In partnership with the company Emulate Inc., we will develop a specialised organ-on-a-chip
model using microfluidic platform technology coupled with optimised biomechanical loading
of the different bone cell types in co-culture with breast cancer cells maintained in a 3D
matrix. We will characterise this new model and validate it against known chemotherapeutics
in terms of phenotypic stability, cancer cell proliferation, migration and invasion and bone
formation and remodelling. To test our hypothesis we will examine cancer cell behaviour with
and without bone cells and mechanical loading. We will then quantify cancer cell behaviour
following treatment with siRNA and pathway antagonists to disrupt osteocyte ciliogenesis
and regulation by cancer cells within the chip. Finally we will test the effect of pharmaceutical
regulation of osteocyte cilia to identify the potential of novel ciliotherapy for treatment of bone
metastasis.
How the results of this research will be used
The project will deliver novel organ-on-a-chip technology for investigating bone metastasis in
breast cancer and will be used here to examine the hypothesis that bone cells regulate
metastasis in response to mechanical loading and modulated by changes in primary cilia.
This is likely to be transferable to other cancers involving bone metastasis and may lead to
novel ciliotherapies. The chip may also be modified for use with autologous cells making it
suitable for personalised medicine therapies and will provide a scalable platform for testing
new cancer therapeutics.
A common site for invasive ductal carcinomas (IDC) metastasis is bone, affecting about 70%
of patients. Once metastasis to bone has occurred the five-year survival rate drops from
99% to 29%. How breast cancer metastasises to bone is poorly understood, partly because
of the lack of appropriate models. Organ-on-a-chip technology is a new branch of
bioengineering which may be used to accurately recapitulate bone metastasis by combining
multiple cell types in a microfluidic chip with circulating media and physiological
biomechanical forces. However a reliable breast cancer bone metastasis chip is currently not
available.
Aims
The aim of this study is to develop an organ-on-a-chip model of bone metastasis in breast
cancer and to use this to test the hypothesis that the interaction between cancer cells and
osteocytes controls the metastatic niche and tumour development. We hypothesize that this
process is regulated by physiological biomechanical loading of bone and osteocyte primary
cilia.
Methods
In partnership with the company Emulate Inc., we will develop a specialised organ-on-a-chip
model using microfluidic platform technology coupled with optimised biomechanical loading
of the different bone cell types in co-culture with breast cancer cells maintained in a 3D
matrix. We will characterise this new model and validate it against known chemotherapeutics
in terms of phenotypic stability, cancer cell proliferation, migration and invasion and bone
formation and remodelling. To test our hypothesis we will examine cancer cell behaviour with
and without bone cells and mechanical loading. We will then quantify cancer cell behaviour
following treatment with siRNA and pathway antagonists to disrupt osteocyte ciliogenesis
and regulation by cancer cells within the chip. Finally we will test the effect of pharmaceutical
regulation of osteocyte cilia to identify the potential of novel ciliotherapy for treatment of bone
metastasis.
How the results of this research will be used
The project will deliver novel organ-on-a-chip technology for investigating bone metastasis in
breast cancer and will be used here to examine the hypothesis that bone cells regulate
metastasis in response to mechanical loading and modulated by changes in primary cilia.
This is likely to be transferable to other cancers involving bone metastasis and may lead to
novel ciliotherapies. The chip may also be modified for use with autologous cells making it
suitable for personalised medicine therapies and will provide a scalable platform for testing
new cancer therapeutics.